Application Note: Quantitation of intact therapeutic protein in plasma matrix by LC/MS
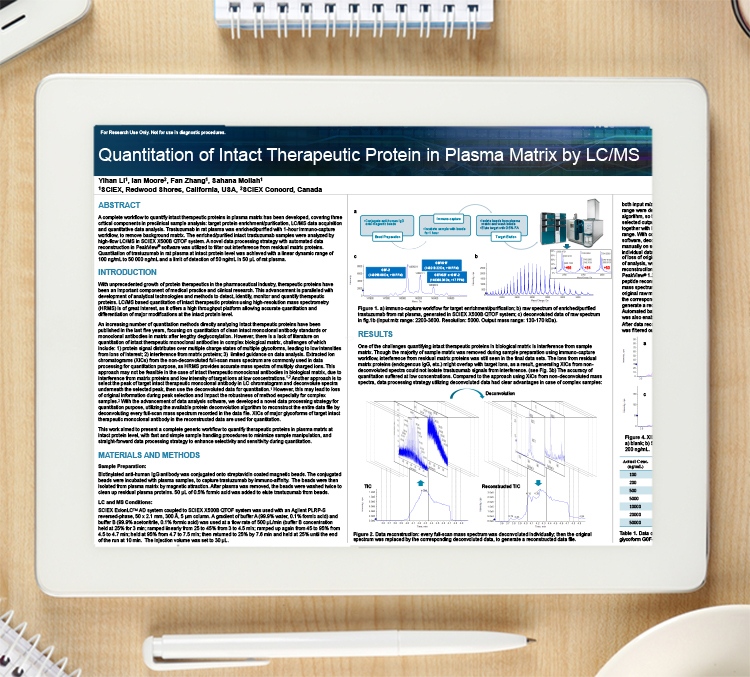
A complete workflow to quantify intact therapeutic proteins in plasma matrix has been developed, covering three critical components in preclinical sample analysis: target protein enrichment/purification, LC/MS data acquisition and quantitative data analysis.