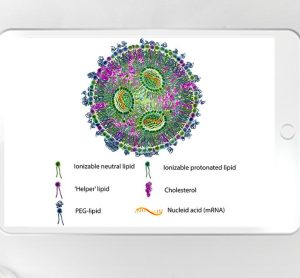
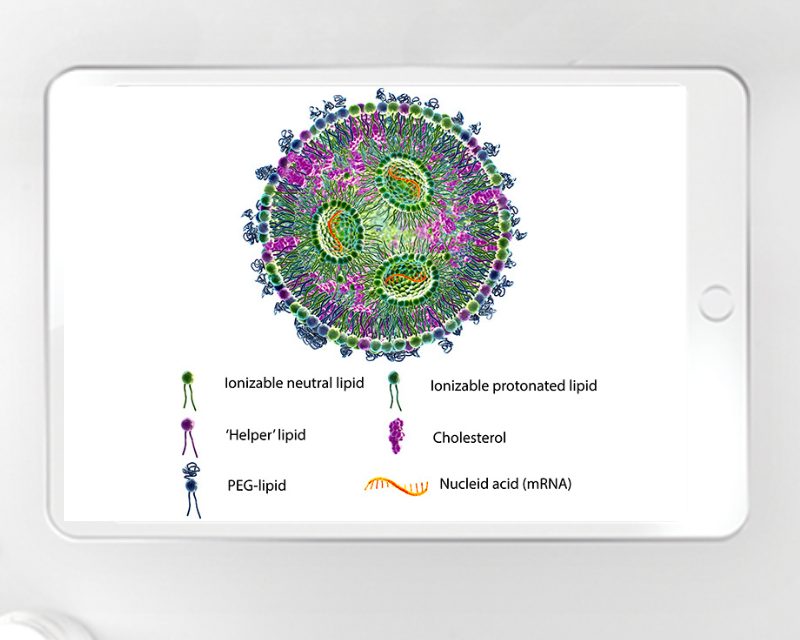
The Critical Role of CCI in Drug Development and Manufacturing
This whitepaper provides a detailed examination of Lonza’s holistic, data-driven approach to CCI, developed in collaboration with industry experts and applied across our global network for parenteral combination products.