On-demand webinar: Pyrogenic Risk in Pharma Process Webinar – from bioburden to pyrogens
Posted: 28 June 2019 | Merck | No comments yet
Microbial risk in pharmaceutical process is not limited to living microorganisms and intact microbial cells.
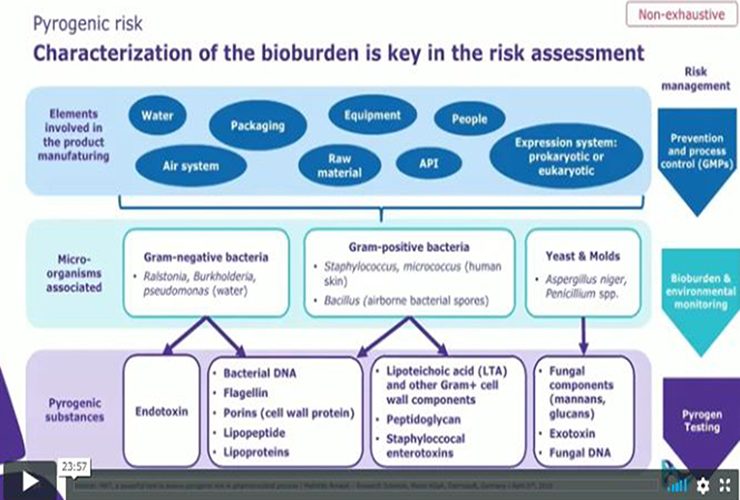

Subcellular components from microorganisms remaining from the production process can be source of pyrogens, compromising product quality and patient safety as these substances are not eliminated by classical filtration or sterilization steps.
Related topics
Microbial Biological Manufacturing, Microbial Detection, Production








