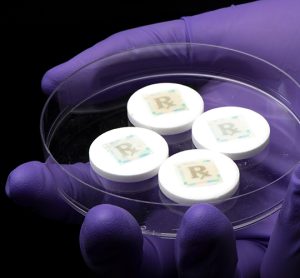
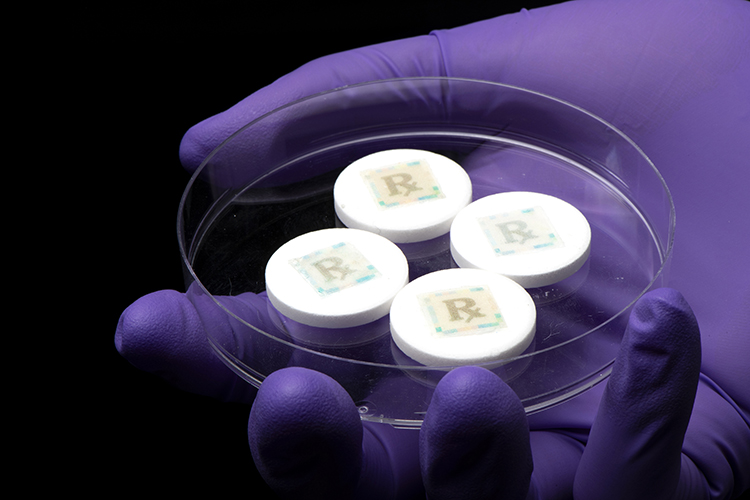
Securing every dose with an edible security technology for safe medicines
The problem of counterfeit medicines is becoming a tremendous burden to society. An edible security tag affixed to each medicine can provide track and trace measures with dose information and on-dose (in-dose) authentication. Furthermore, it serves as a last line of defence against illicit pharmaceutical products. Young Kim, Associate Head…