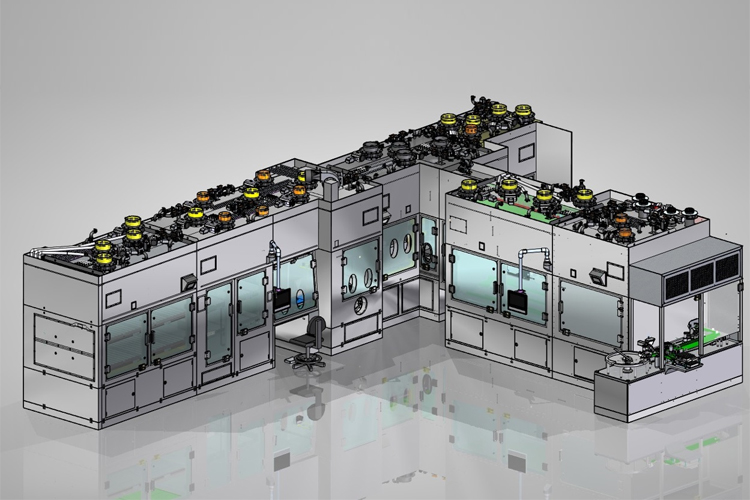
Microbiology In-Depth Focus 2019
The work of a pharmaceutical scientist requires precision, attention to detail and is of vital importance to the safety of products and ultimately patients. This section features insights from experienced scientists who’ve worked extensively in microbiological analysis, developing tests, methods and standards that help establish a foundation for the production…