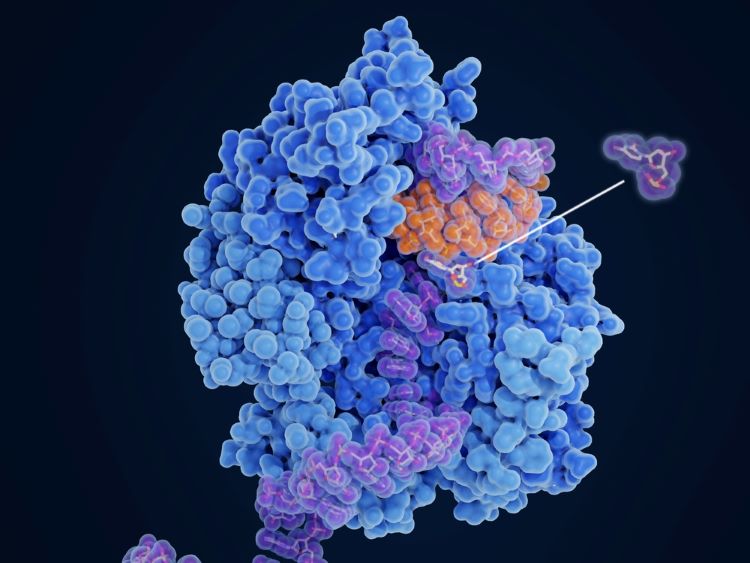
Optimising reverse-phase chromatography for molnupiravir production
The research offers insight into optimising chromatographic separation outcomes through precise control of measures such as flowrate and column length, supporting potential for drug development.