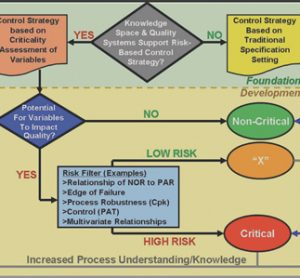
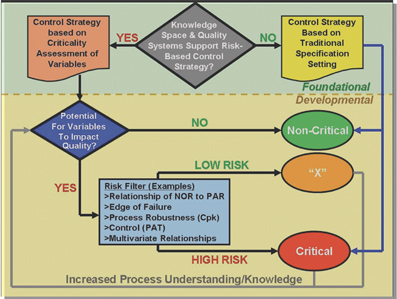
QbD and PAT: From Science to Compliance
30 July 2009 | By Pedro E. Hernandez-Abad, Associate Director; Jun Huang, Principal PAT Scientist II and Saly Romero-Torres, Principal PAT Scientist, both Wyeth Pharmaceuticals
Boards of health like the Food and Drug Administration and European Medicines Agency and ICH guidelines Q8, Q9 and Q10, provide a framework for Quality by Design (QbD) that fully integrates drug substance and drug product development with the principles of Quality Risk Management (QRM), Process Analytical Technology (PAT) and…