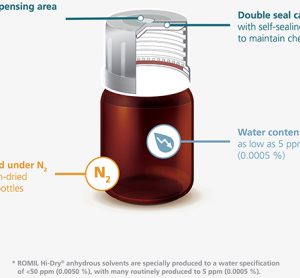
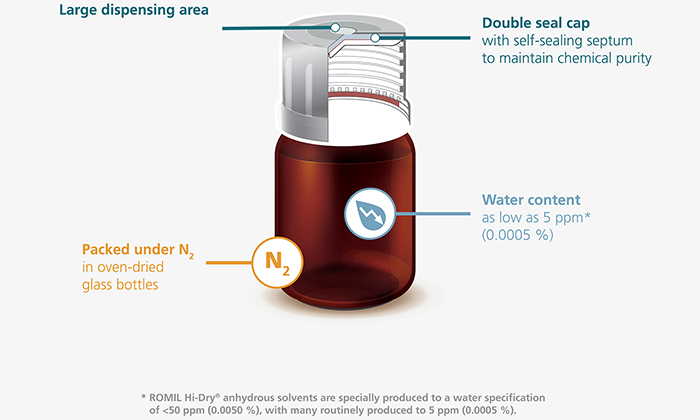
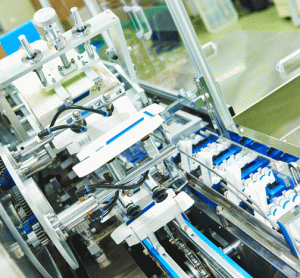
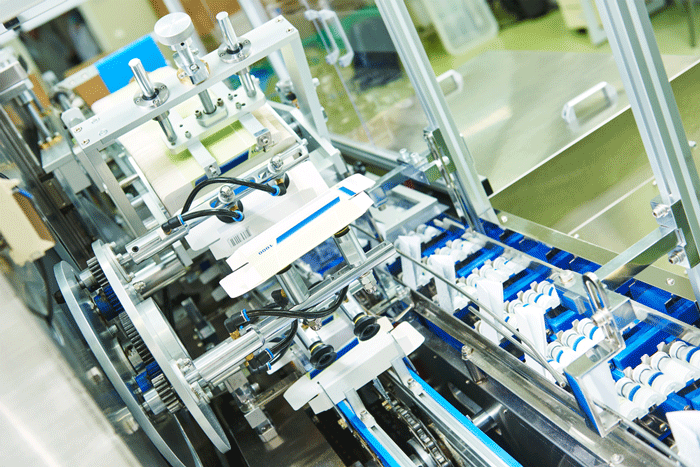
Gore to debut GORE™ ImproJect™ plunger for sensitive biologics in pre-filled syringes
NEWARK, Del. – W.L. Gore & Associates, Inc. (“Gore”), a global materials science company, will introduce its new GORE™ ImproJect™ Plunger at Pharmapack 2018, Europe's dedicated pharmaceutical packaging and drug delivery event. Gore will be in Booth A12 at the exhibition, to be held February 7 and 8, 2018, at…