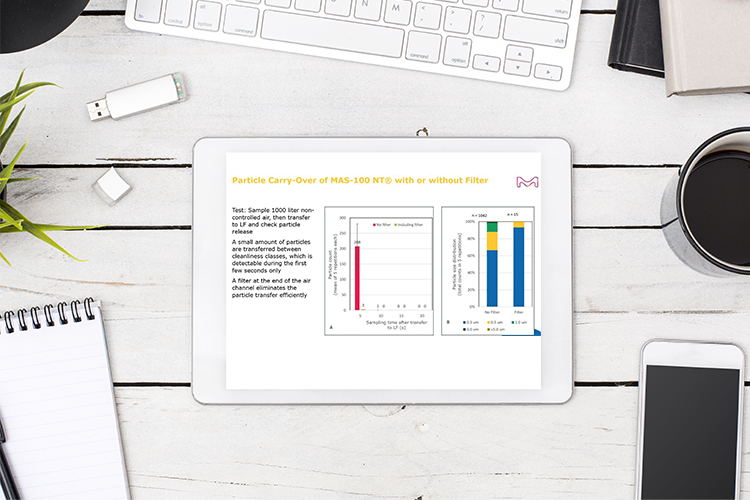
Release of sterile medicinal products – looking at the focal points
There are numerous risks and regulatory requirements that must be considered to ensure appropriate contamination control of sterile medicinal products. Tim Sandle discusses the complexities of sterility assurance and provides guidance for manufacturers to ensure that appropriate risk management processes are in place.