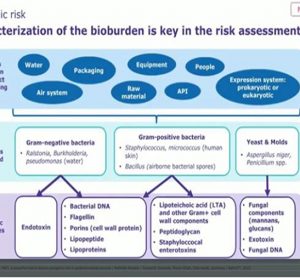
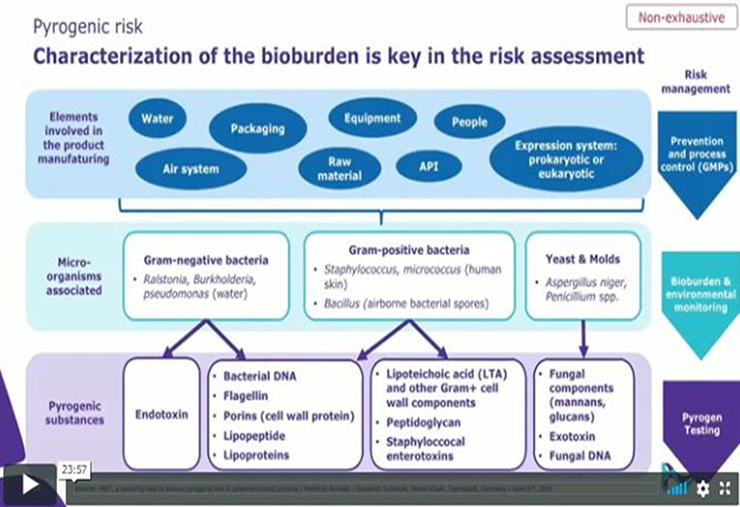
Quality control solutions that fuel confident decisions
As the partner of choice for managing microbial quality control, Charles River’s products and services facilitate confident and objective decision-making, ensuring the integrity of your microbial data, reducing risk, building efficiency and improving your bottom line, while assisting in the journey to bring products to market.