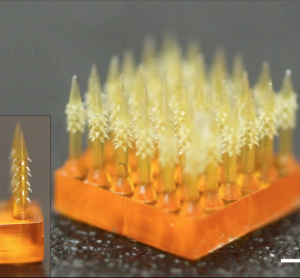
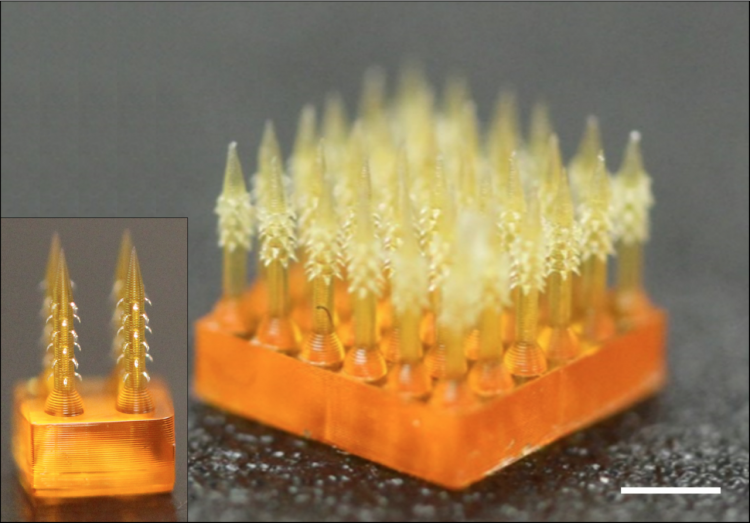
Safe’n’Spray™, the smart electronic concept device with child-resistant and locking features, wins the ‘Best Innovation In Drug Delivery Device’ Award at Pharmapack 2020
A team of industry experts awarded Nemera’s Safe’n’Spray with a prestigious Pharmapack Award for ‘Best Innovation in Drug Delivery Device’.