Mass spectrometry-based metabolics – an exclusive online-only article from Tun-Li Shen at Brown University
3 July 2014 | By Tun-Li Shen, Brown University
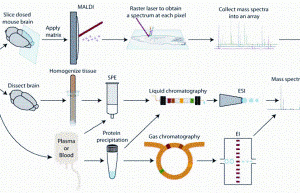
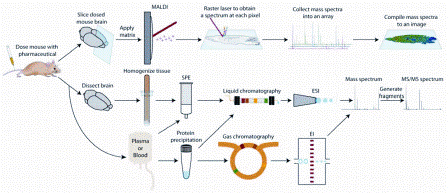
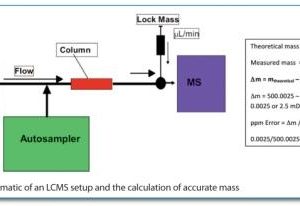
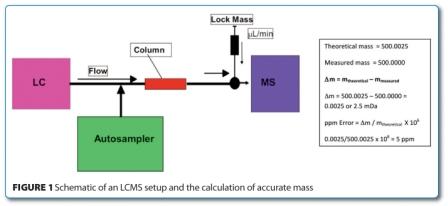
Tun-Li Shen provides a brief overview of the gas chromatography mass spectrometry (GC-MS) and liquid chromatography mass spectrometry (LC-MS) instrumentation platforms used in untargeted metabolomics studies...