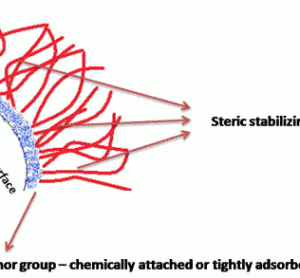
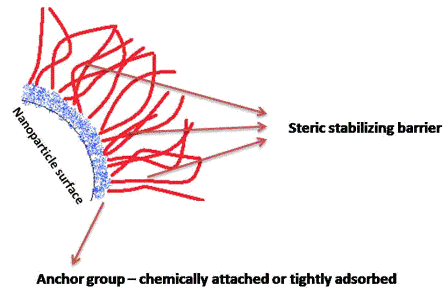
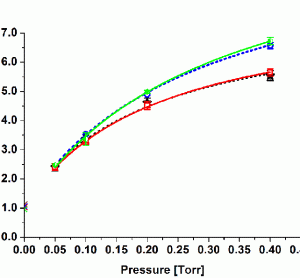
PAT Series: Recent achievements in NIR-based on-line monitoring of lyophilisation processes
30 June 2016 | By Adrian Funke - Bayer Pharma AG / Reinhard Gross, Stephan Tosch and Albert Tulke - Bayer Technology GmbH
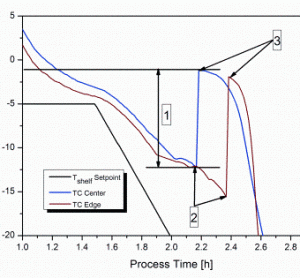
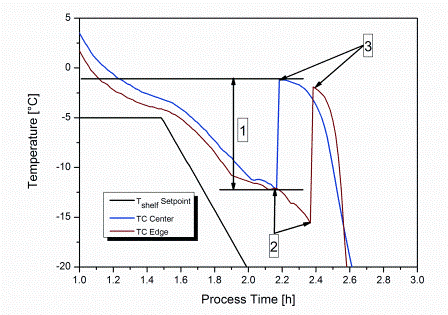
Process analytical technology (PAT), namely near-infrared (NIR) and Raman spectroscopy, has already been shown to be a useful tool for monitoring, analysing and optimising the complex process of lyophilisation. The latter process is especially challenging in the case of biopharmaceutical formulations due to the instability of active ingredients, leading to…