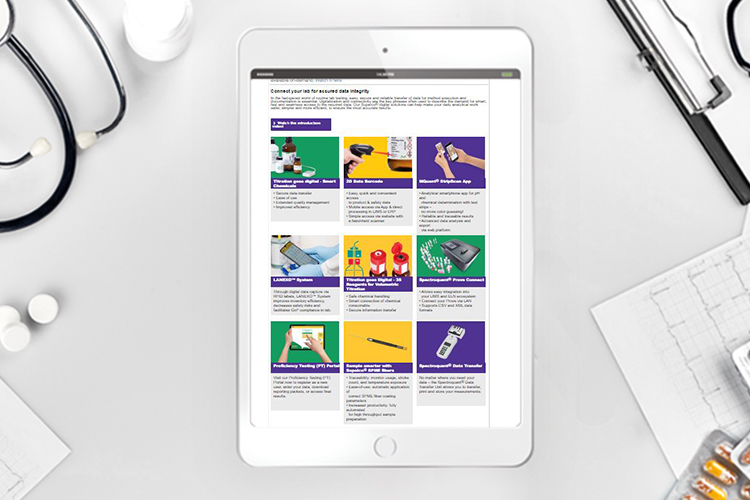
Why pharma firms would benefit from a modular approach to working with product labelling
According to David Bennett, securing and building trust in the label, brand and process of a highly regulated industry player in a complex age will demand a new approach.