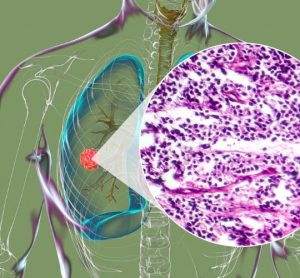
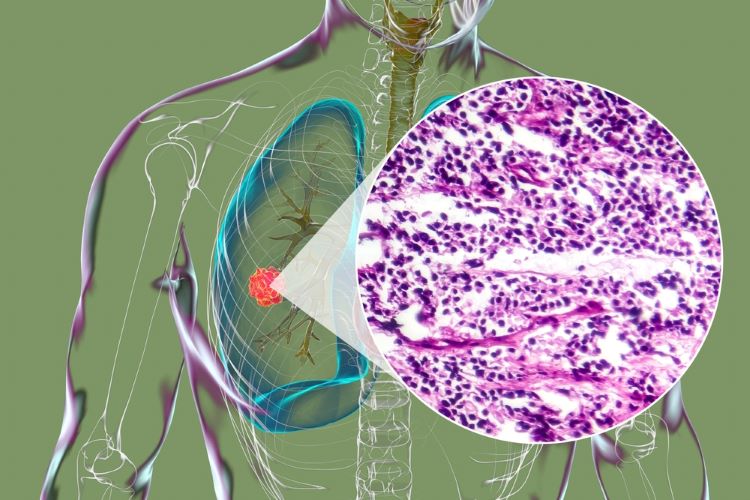
Boehringer agrees new partnership to advance first-in-class precision cancer therapies
The collaboration between Boehringer Ingelheim and Tessellate Bio seeks to address availability of targeted medicines for a cancer type shown to be difficult to treat.