NK cell immunotherapy: what’s next in clinical development?
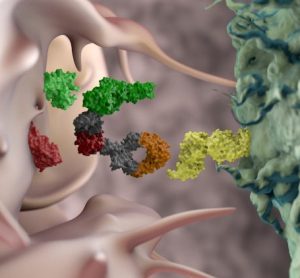
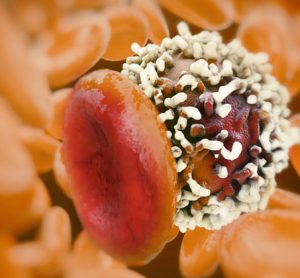
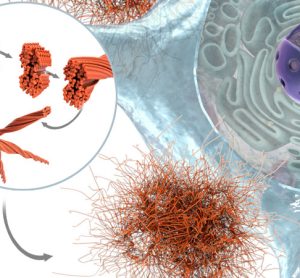
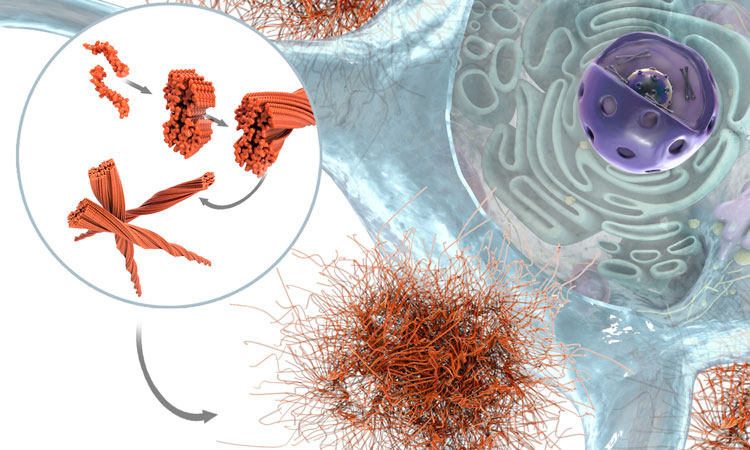
In this interview, Innate Pharma’s Yannis Morel, Executive Vice President of product portfolio strategy and business development, delves into the unique advantages of using multi-specific antibodies capable of engaging NK cells against tumours for oncology indications, and shares key data from the company’s ongoing and recent clinical trials.