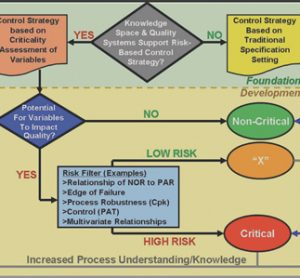
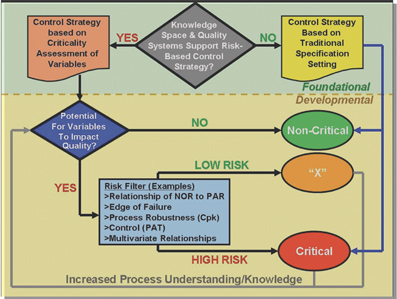
Review of regulatory changes impacting on pharamceutical microbiology
23 December 2014 | By Dr Tim Sandle, Bio Products Laboratory
This article surveys some of the recent developments in regulatory requirements and standards that have taken place during the past 12 months, highlighting those aspects that are of relevance to pharmaceutical microbiology...