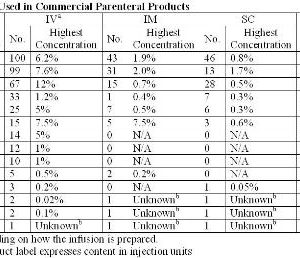
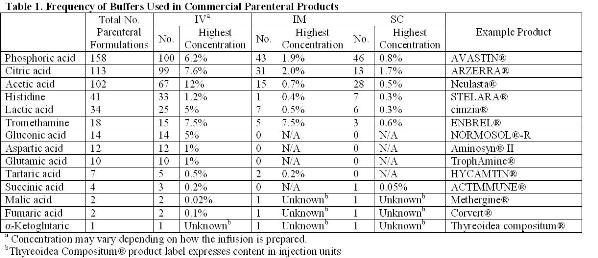
Dynamic vapour sorption of freeze-dried pharmaceuticals
22 October 2015 | By Claudia Kunz and Henning Gieseler, Department of Pharmaceutics, University of Erlangen
Freeze drying is gaining in importance as the number of biopharmaceuticals that are unstable in a solution increases. According to recent reports, a growth of 10% may be expected for freeze-dried products in the next 10 years. The technique offers the opportunity to gently dry temperature-sensitive drugs such as proteins…