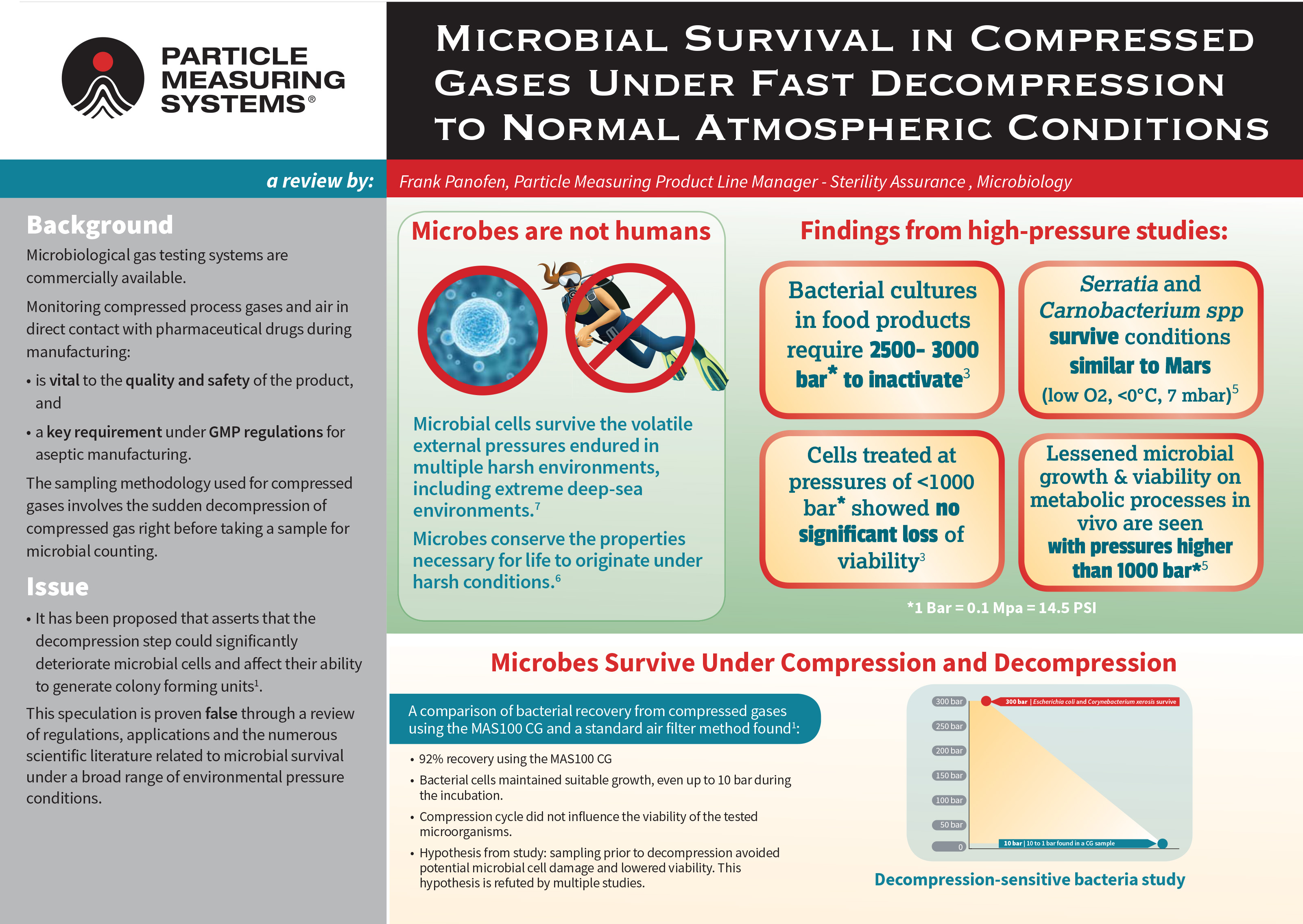
Poster: Microbial survival in compressed gases under fast decompression to normal atmospheric conditions
This scientific poster reviews numerous scientific literature related to microbial survival under a broad range of environmental pressure conditions, and the resilience of microorganisms under variable compression and decompression environments.