Implementing electronic laboratory notebooks to improve the efficiency of pre-clinical drug discovery
13 December 2011 | By Sheraz Gul, Vice President and Head of Biology, European ScreeningPort GmbH
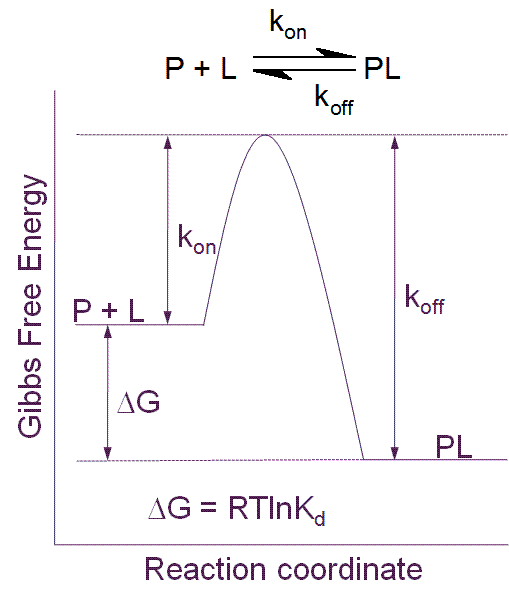
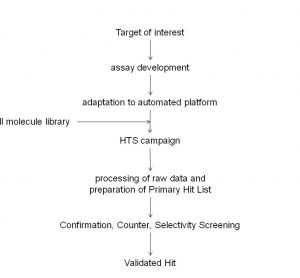
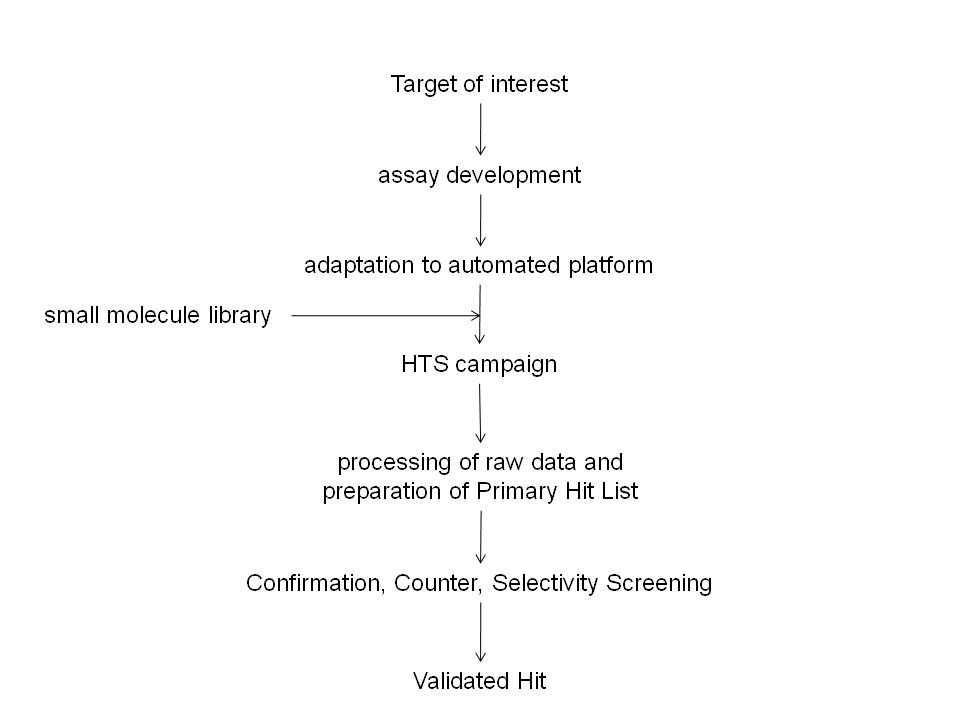
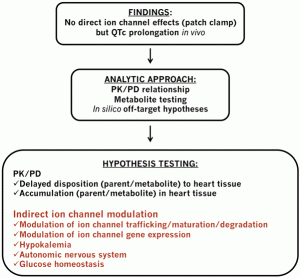
The pre-clinical phase of drug discovery spans a period in the region of five years and requires contributions from multi-disciplinary teams often working at different sites. These teams can generate significant amounts of data which are processed using standard as well as specialist software. The recording of a substantial amount…