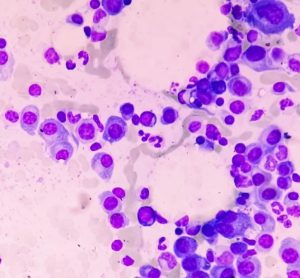
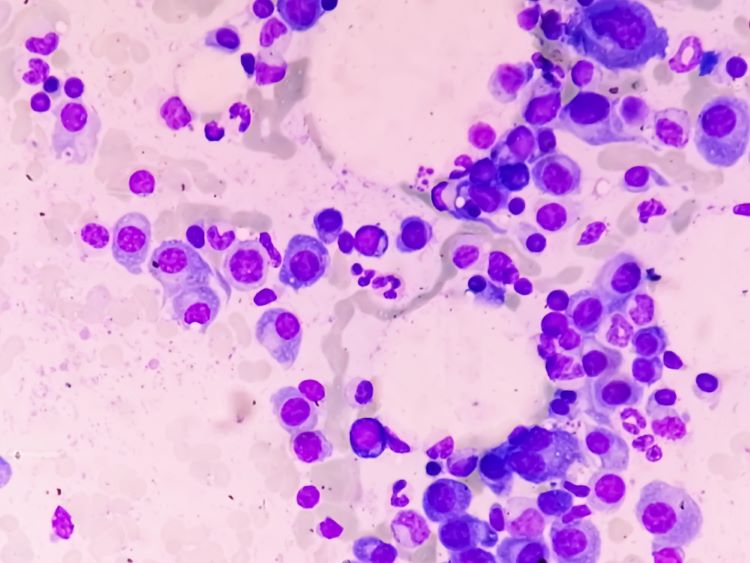
Innovative nanoparticle-based technique could advance pharmaceutical formulations
The paper highlights a new analytical method that provides potential for sustainable use of nanomaterials and unique benefits compared to other spectrofluorimetric techniques.