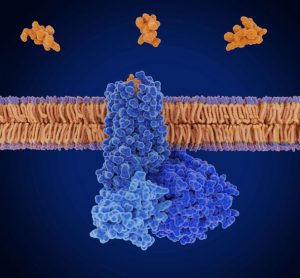
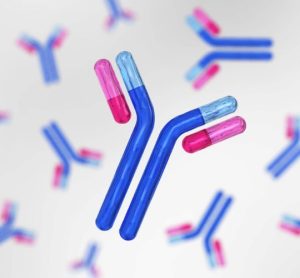
Restoring regulatory excellence “central” to UK life science competitiveness
The Association of the British Pharmaceutical Industry (ABPI) asserts that the recommendations will help generate confidence and predictability in medicine regulation in the UK.