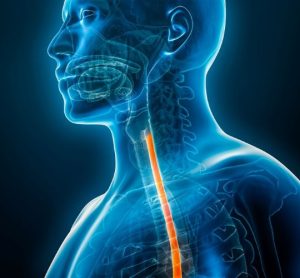
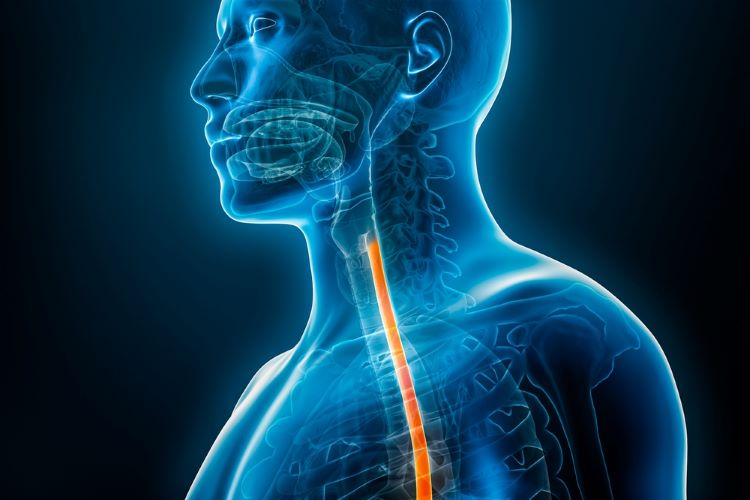
Eosinophilic esophagitis medicine approval expanded
Approval of the first treatment for patients one year old and over with eosinophilic esophagitis (EoE) was based on trial data showing a greater proportion of children given Dupixent achieved histological remission compared to placebo.