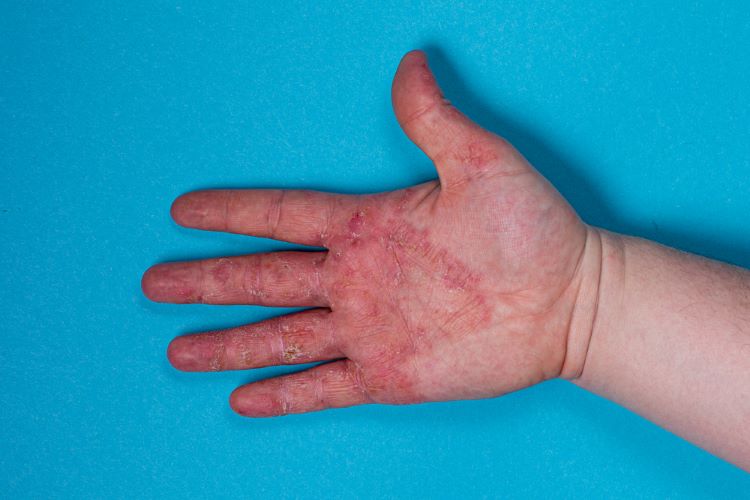
New guidance to aid cost-effectiveness analysis of new drugs
The report aims to support development of economic models for health technology assessment (HTA) decision making, as there has been “little change in guidance on the use of surrogate endpoints in HTA since 2018”.