Scrubbing up the environment
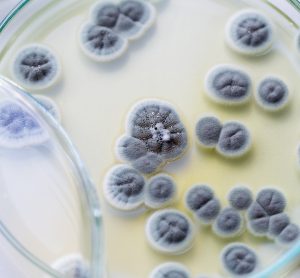
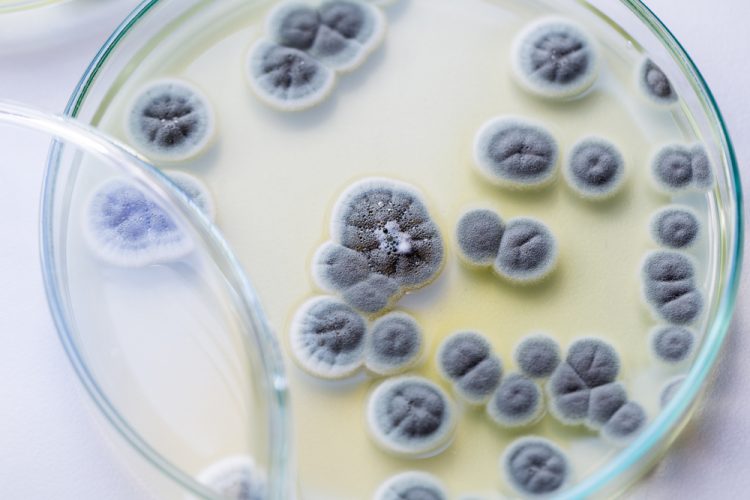
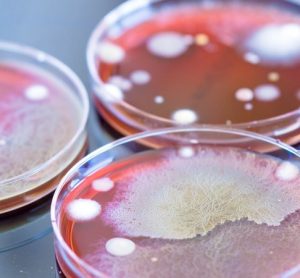
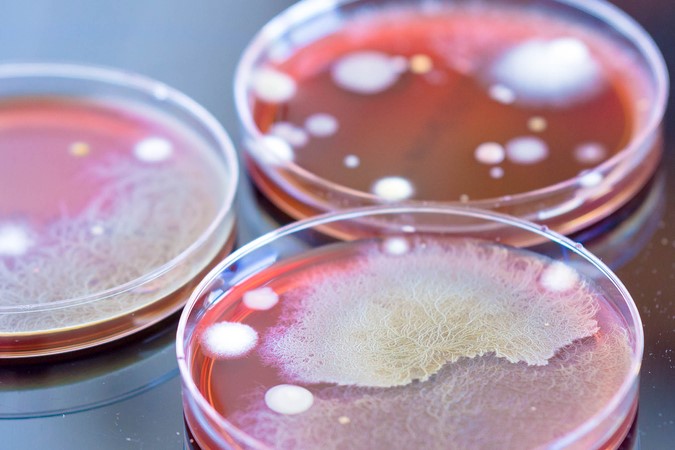
Here, colleagues from University Hospitals Bristol and Weston NHS Foundation Trust (UHBW) share the beneficial impact of reduced cleanroom environmental contamination following an upgrade to their facilities and procedures.