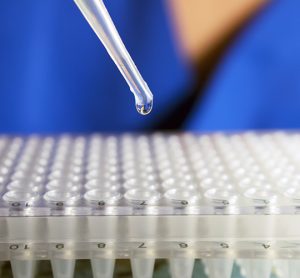
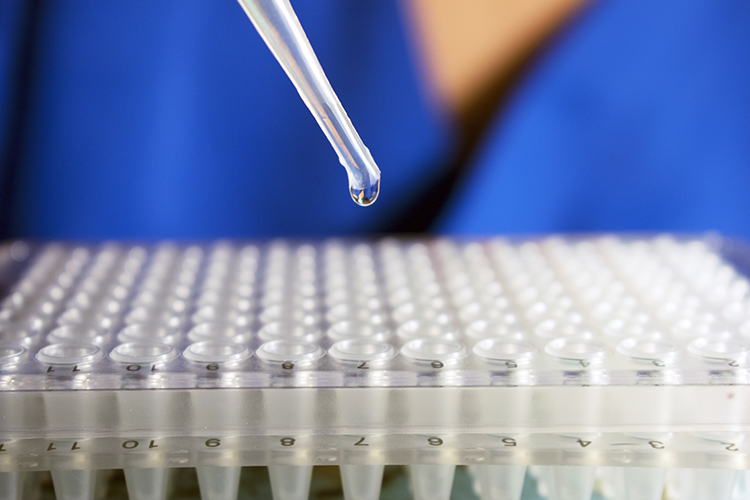
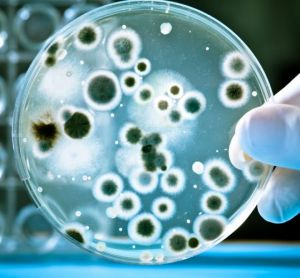
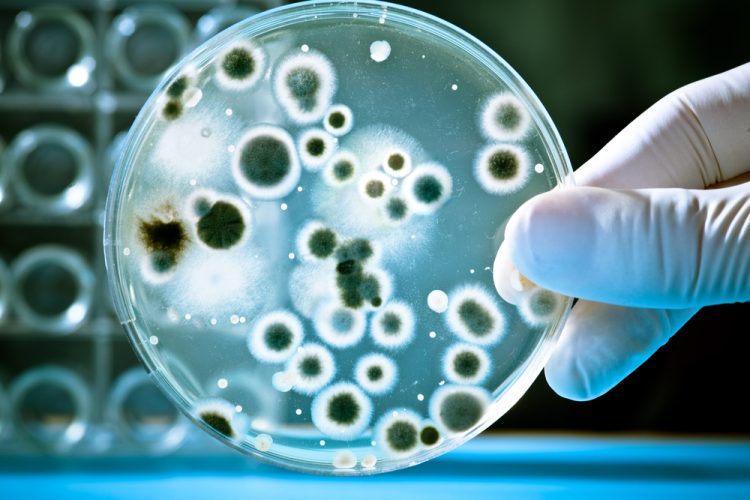
Implementing a real-time PCR-based method for sterility release testing of ATMPs
23 December 2022 | By Sartorius
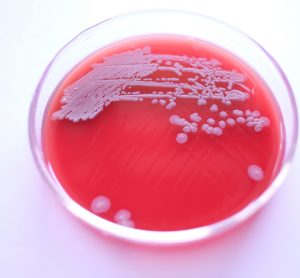
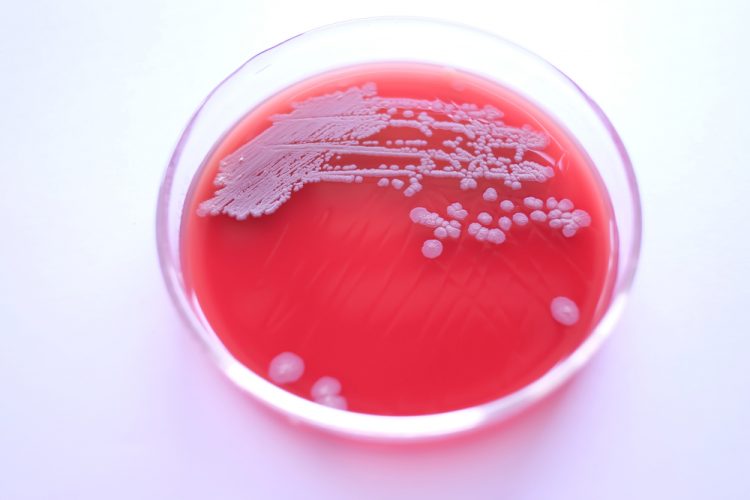
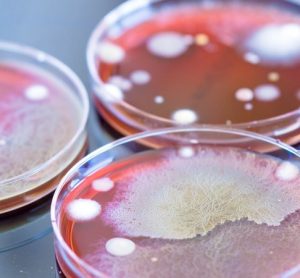
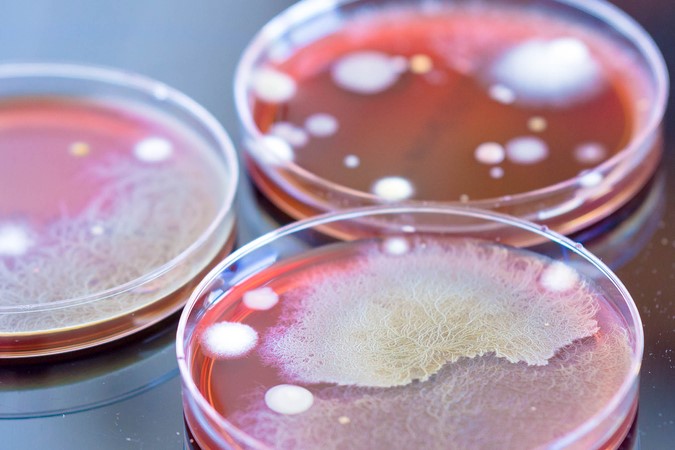
Dr Alexandra Mueller-Scholz, Sartorius, and Yasmin Heynen, Molecular Development at Labor LS, discuss rapid sterility testing for ATMPs using a real-time PCR-based method.