FDA approves IMBRUVICA® (ibrutinib) for treatment of chronic lymphocytic leukaemia and small lymphocytic lymphoma
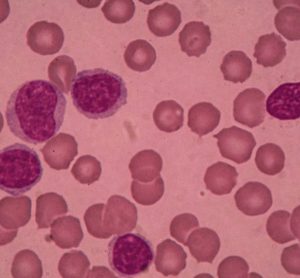
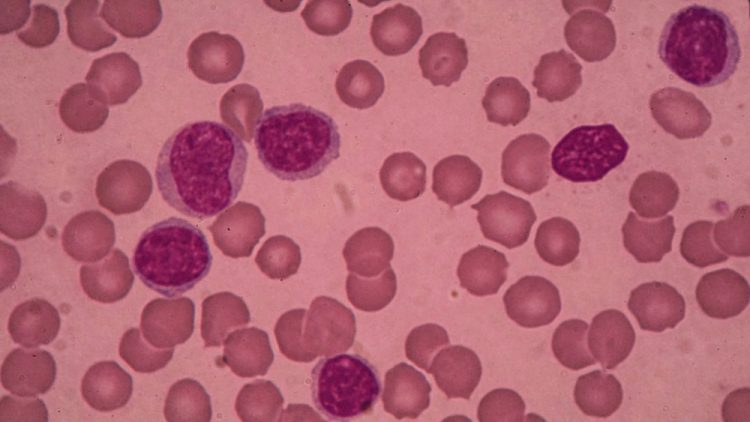
IMBRUVICA (ibrutinib), in combination with rituximab, has been given approval in the US for the treatment of chronic lymphocytic leukaemia and small lymphocytic lymphoma.