EC approves Type II variation for Takeda’s Adcetris
22 January 2016 | By Victoria White
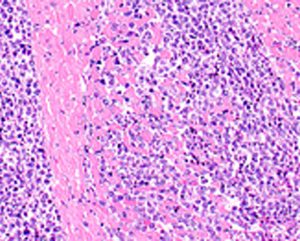
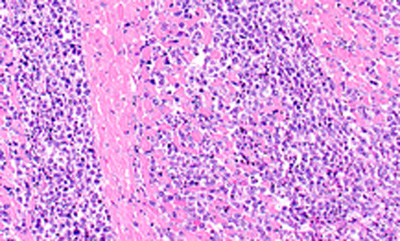
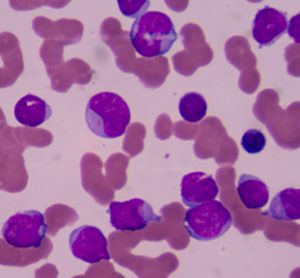
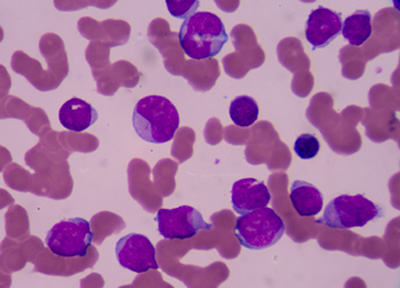
The EC has approved the Type II variation for Adcetris to include data on the retreatment of adult patients with R/R Hodgkin lymphoma or R/R sALCL who previously responded to Adcetris and who later relapse....