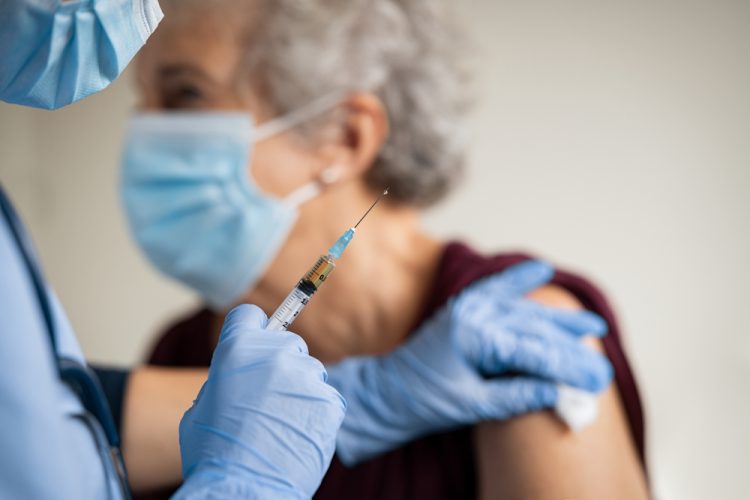
Disinformation and safety concerns could prevent successful COVID-19 vaccination campaigns
Research suggests at least a fifth of the US population are resistant to receiving a COVID-19 vaccine, reports suggest this could be due to disinformation and safety concerns.