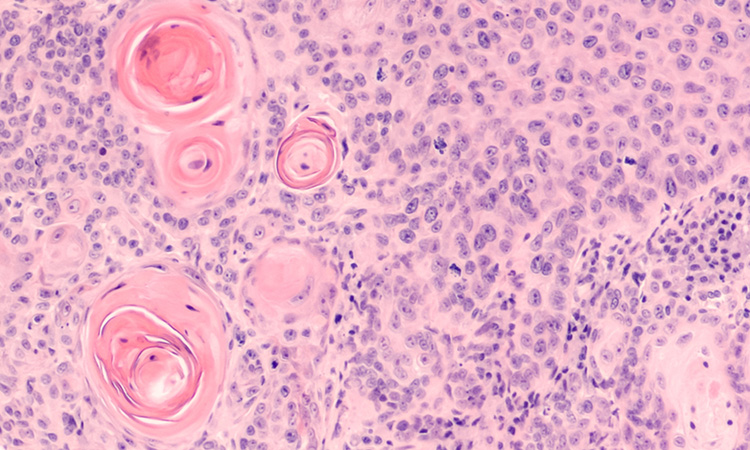
Marketing of Zynteglo suspended as precaution due to potential cancer risk
bluebird bio has chosen to suspend sales of Zynteglo while they investigate whether safety concerns identified with a related investigational gene therapy may apply to the licenced medicine.











![Jazz Pharmaceuticals Sign with Logo [Credit: Michael Vi / Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Jazz-pharmaceuticals-300x278.jpg)
![Jazz Pharmaceuticals Sign with Logo [Credit: Michael Vi / Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Jazz-pharmaceuticals-e1612534960797.jpg)