New NDA Infographic highlights state of drug approvals in 2014: EU vs USA
6 May 2015 | By Victoria White
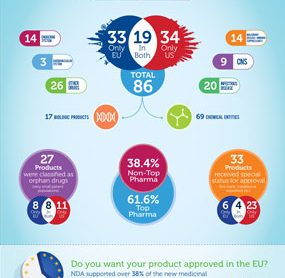
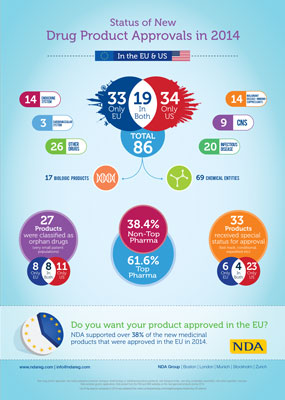
NDA Group's new Infographic looks at drug approvals in 2014 and highlights the fact that nearly 40% of approved products came from non top pharma companies...