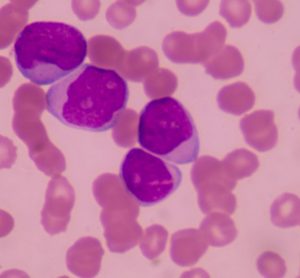
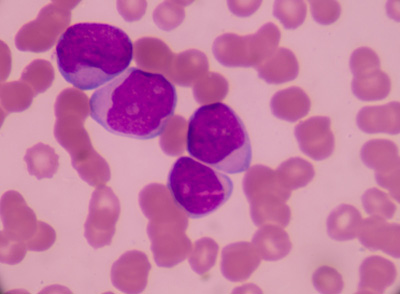
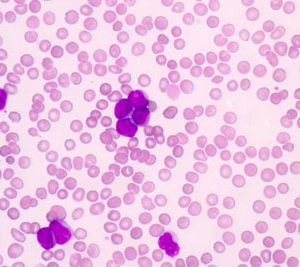
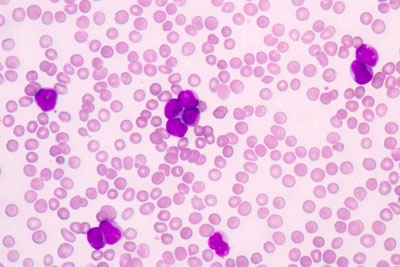
EC approves Vidaza as a new treatment for elderly patients with AML
30 October 2015 | By Victoria White
Dr Hervé Dombret says the 'announcement brings hope to patients with AML, particularly the elderly and more frail patients who cannot undergo intensive therapies'...