Bonesupport enrols first patient in injectable bone graft substitute trial
22 May 2017 | By Niamh Marriott, Junior Editor
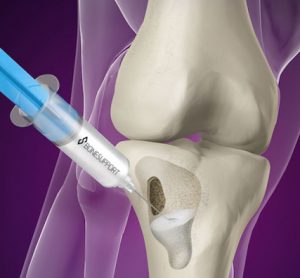
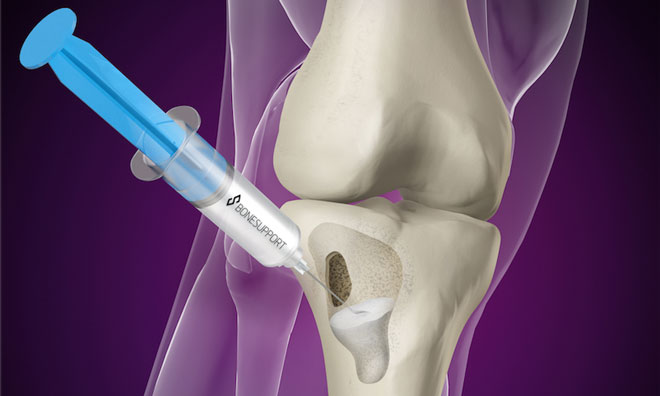
Bonesupport, the Scandinavian medical technology company, has enrolled their first patient at the University of Texas Health Science Center at San Antonio into the company’s pivotal Investigational Device Exemption (IDE) trial. Cerament G is an injectable antibiotic-eluting bone graft substitute that has proven remodelling capabilities and provides local sustained delivery…