AstraZeneca reports new data for osimertinib in NSCLC
15 April 2016 | By Victoria White, Digital Content Producer
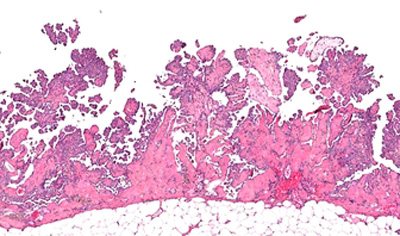
AstraZeneca has reported new Phase I extended follow-up data on osimertinib in both first- and second-line treatment of patients with non-small cell lung cancer (NSCLC).