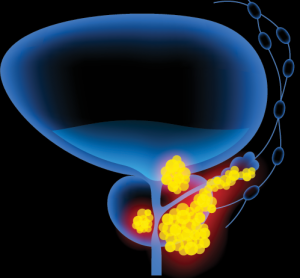
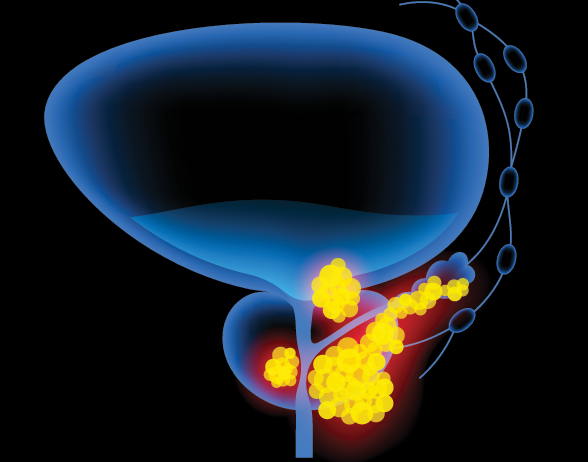
NICE recommends combination therapy for prostate cancer
Lynparza (olaparib), which is being co-developed by AstraZeneca and MSD, showed clinically meaningful benefit when used with abiraterone and prednisone or prednisolone to treat hormone-relapsed metastatic prostate cancer.