Grazoprevir/Elbasvir, Merck’s investigational chronic hepatitis C therapy, granted FDA Breakthrough Therapy designations
9 April 2015 | By Victoria White
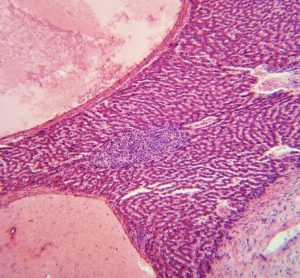
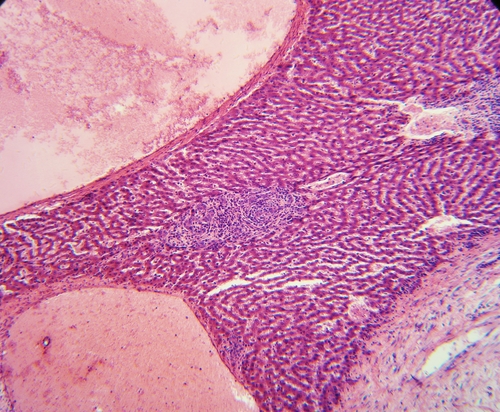
Merck has announced that grazoprevir/elbasvir has received two new Breakthrough Therapy designations from the U.S. Food and Drug Administration (FDA)...