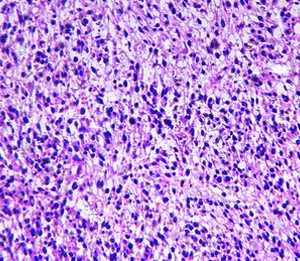
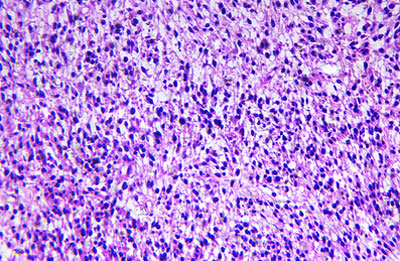
New test could help personalise treatment for rhabdomyosarcoma
15 October 2015 | By Victoria White
In a study, examining the activity of only five genes in a sample of the tumour was enough to identify high-risk children who might benefit from more intensive treatment...