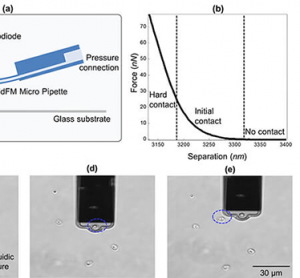
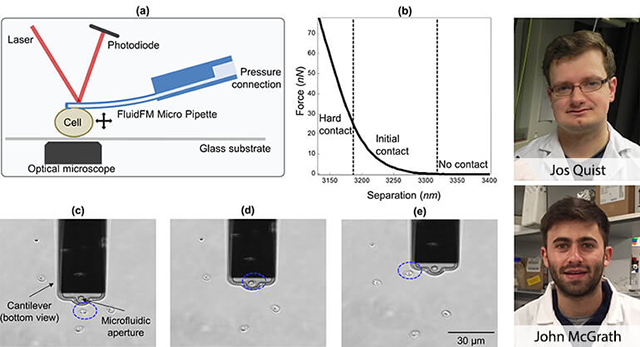
NICE draft guidance recommends treatments for breast and lung cancer
10 November 2016 | By Niamh Louise Marriott, Digital Content Producer
NICE recommends 2 cancer drugs for routine use on the NHS as part of its programme to appraise treatments that were available on the Cancer Drugs Fund...