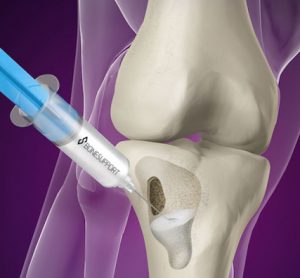
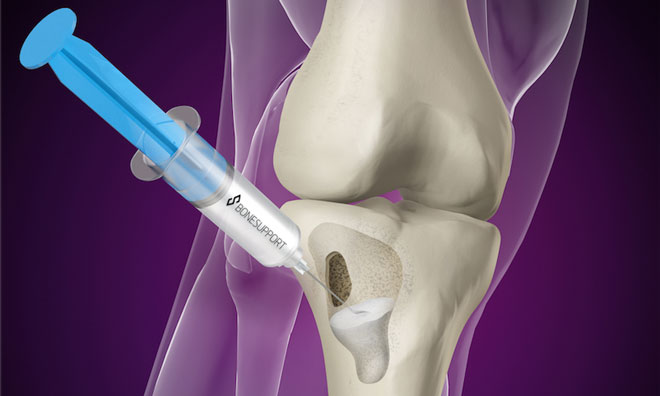
Better, cheaper healthcare with dry blood samples
24 May 2017 | By Niamh Marriott, Junior Editor
In a new study, Uppsala University researchers have successfully measured 92 different proteins in millimetre-sized circles punched out of dried samples...