FDA revokes emergency use authorisation for chloroquine and hydroxychloroquine as COVID-19 treatments
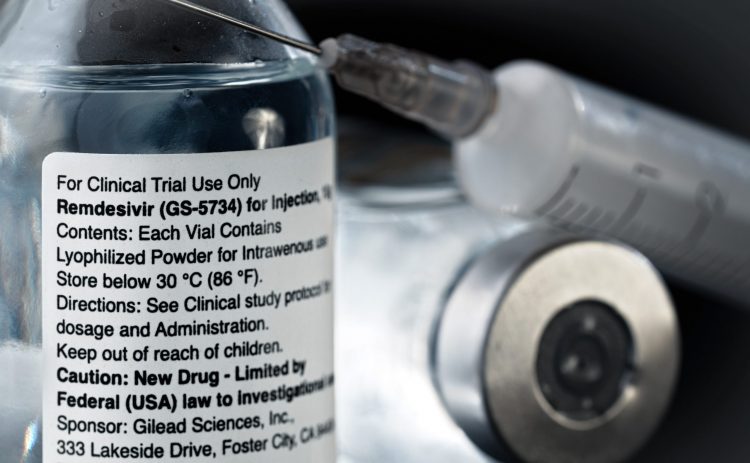
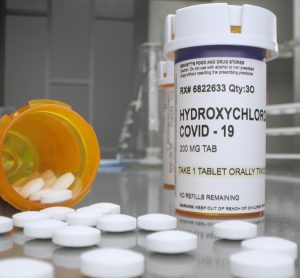
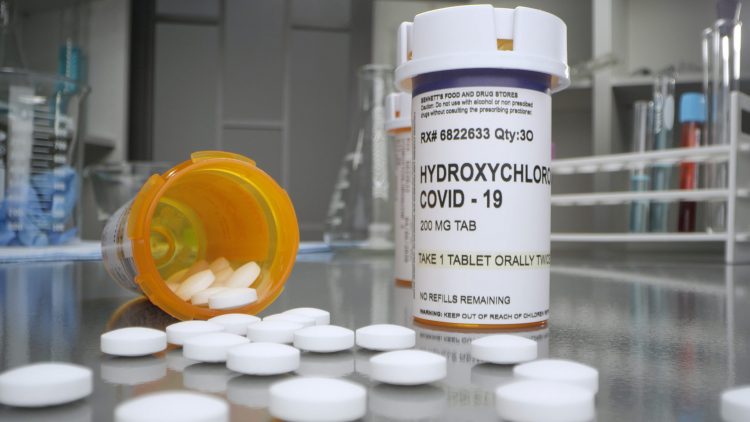
Following ongoing analysis and emerging scientific data, the FDA has taken away its emergency use authorisation for chloroquine and hydroxychloroquine, finding they are unlikely to be effective as COVID-19 treatments.