EMA surveys pharma companies on their preparedness for Brexit
Posted: 26 January 2018 | Dr Zara Kassam (European Pharmaceutical Review) | No comments yet
The EMA is launching a survey to gather information from companies on their Brexit preparedness plans and identify any particular concerns with regard to medicines supply that may impact public or animal health…


The European Medicines Agency (EMA) is launching a survey to gather information from companies on their Brexit preparedness plans and identify any particular concerns with regard to medicines supply that may impact public or animal health.
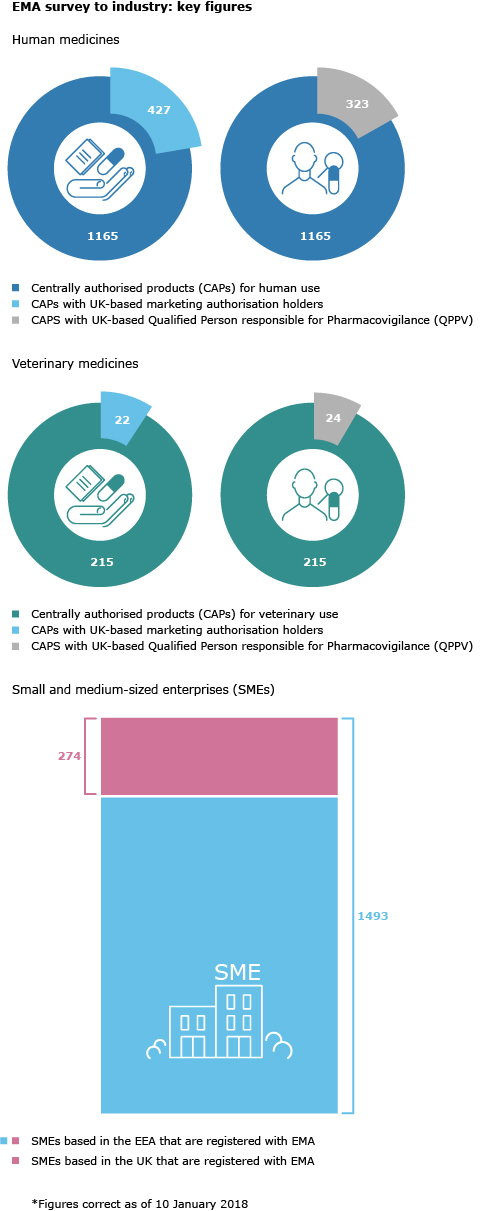
EMA is consulting marketing authorisation holders of centrally authorised medicines that are located in the United Kingdom (UK), or who have quality control, batch release, and/or import manufacturing sites or a qualified person for pharmacovigilance(QPPV) or pharmacovigilance system master file (PSMF) in the UK, on their plans to submit transfers, notifications or variations to their marketing authorisations in the context of the UK’s withdrawal from the European Union (EU).
The aim of this survey is twofold. Firstly, to identify those companies where there is a need for concerted action to address medicines supply concerns due to Brexit in order to protect human and animal health, and secondly to help the Agency and the European Commission plan resources in the areas where these submissions will be processed. Information from the survey will also be used to inform next steps in Brexit preparedness for EMA, the European Commissions and the European medicines regulatory network.
The survey is being sent directly to holders of a centralised marketing authorisation for human and veterinary medicines who are located in the UK, or who have an important part of their site operations in the country. The deadline for completion of the questionnaire is 9 February 2018.
The survey will also serve to stimulate those companies who have not yet taken any action to start planning for any regulatory steps required for their centrally authorised products to remain on the EU market post-Brexit in order to minimise disruption to medicines supply and avoid shortages.
Findings and recommendations from the survey will be shared with the European Commission and presented to EMA’s Management Board. A high-level summary of the overall results of the survey will be published on the EMA website.
Further surveys may be carried out by the EU national competent authorities in relation to nationally authorised products. Companies should check EMA’s dedicated webpage regularly for guidance on the consequences of Brexit.

Related topics
Related organisations
European Commission (EC), European medicines regulatory network, The European Medicines Agency (EMA)