FDA grants two orphan drug designations for HCC
Posted: 18 August 2017 | Dr Zara Kassam (European Pharmaceutical Review) | No comments yet
The FDA has granted two orphan drug designations for T cell therapy products for the treatment of hepatocellular carcinoma…

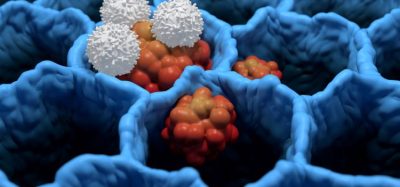
The U.S. Food and Drugs Administration (FDA) has granted two orphan drug designations (ODD) for T cell therapy products for the treatment of hepatocellular carcinoma (HCC).
Biotech company Lion TCR Pte Ltd, are developing two product candidates — HBV specific TCR redirected T cell therapies against HCC with transient Messenger RNA and a longer lasting DNA transduction technology.
The FDA Orphan drug designations are granted to drugs and biologics intended for rare diseases that affect fewer than 200,000 people in the U.S, and provides incentives that may include tax credits trials and user fee waivers, for products that treat diseases or conditions which cannot be satisfactorily treated by available alternative drugs. The FDA may grant in condition approval for marketing after product’s completion of phase II pivotal clinical trial.
This designation also entitles Lion TCR to a seven-year period of marketing exclusivity in the U.S upon issuance of the new drug license.
Lion TCR’s founder and CEO, Dr. Li Lietao said, “Profiting from US FDA’s well established regulatory framework and the ODD grant of our HBV specific TCR-T cell products, we aim to develop the clinical program internationally under FDA Investigational New Drug (IND) covering multiple sites in US, Europe and Asia. It will significantly accelerate our product development and speed up the commercialisation of our products to both US and Asian markets.
HCC is the world’s third leading cause of death due to cancer and the second for China. Globally, 60% of HCC is a result of chronic hepatitis B infection, for the case of China, it is more than 90%. There are 780,000 new HCC cases reported annually, the general prognosis is poor with overall survival rates of 3-5%.