FDA approves Sanofi and Regeneron’s new eczema drug
Posted: 3 April 2017 | Niamh Marriott (European Pharmaceutical Review) | No comments yet
The US Food and Drug Administration (FDA) approved Sanofi and Regeneron‘s Dupixent (dupilumab) injection to treat adults with moderate-to-severe eczema (atopic dermatitis).

Dupixent is intended for patients whose eczema is not controlled adequately by topical therapies, or those for whom topical therapies are not advisable. Dupixent can be used with or without topical corticosteroids.
Dupixent is administered as an injection under the skin. Dupixent’s active ingredient is an antibody (dupilumab) that binds to a protein [interleukin-4 (IL-4) receptor alpha subunit (IL-4Ra)], that causes inflammation. By binding to this protein, Dupixent is able to inhibit the inflammatory response that plays a role in the development of atopic dermatitis.
Supporting studies
The safety and efficacy of Dupixent were established in three placebo-controlled clinical trials with a total of 2,119 adult participants with moderate-to-severe atopic dermatitis not adequately controlled by topical medication(s). Overall, participants who received Dupixent achieved greater response, defined as clear or almost clear skin, and experienced a reduction in itch after 16 weeks of treatment.
About dermatitis
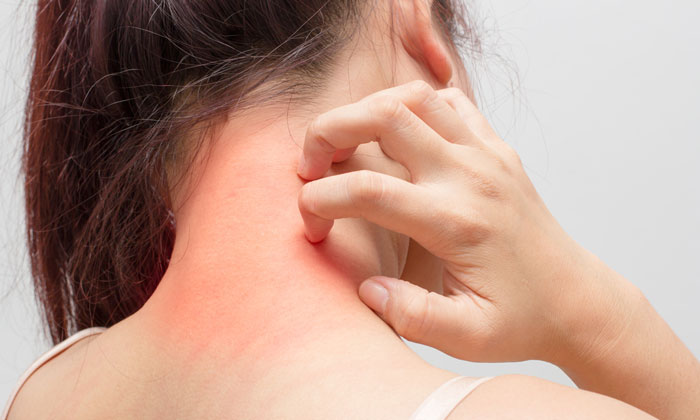
Atopic dermatitis, a chronic inflammatory skin disease, is often referred to as “eczema,” which is a general term for several types of inflammation of the skin. Atopic dermatitis is the most common of the many types of eczema; onset typically begins in childhood and can last through adulthood.
The cause of atopic dermatitis is a combination of genetic, immune and environmental factors. In atopic dermatitis, the skin develops red, scaly and crusted bumps, which are extremely itchy. Scratching leads to swelling, cracking, “weeping” clear fluid, and finally, coarsening and thickening of the skin.
“FDA’s approval of Dupixent demonstrates our commitment to approving new and innovative therapies for patients with skin disease,” said Julie Beitz, M.D., director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research.
“Eczema can cause significant skin irritation and discomfort for patients, so it is important to have a variety of treatment options available to patients, including those patients whose disease is not controlled by topical therapies.”
Adverse effects
Dupixent can cause side effects such as serious allergic reactions and eye problems, such as pink eye (conjunctivitis) and inflammation of the cornea (keratitis). If patients experience new or worsening eye symptoms such as redness, itching, pain or visual changes, they should consult a health care provider. The most common side effects include injection site reactions; cold sores in the mouth or on the lips; and eye and eyelid inflammation, including redness, swelling and itching.
Safety and efficacy
The safety and efficacy of Dupixent have not been established in the treatment of asthma. Patients who also have asthma should not adjust or stop their asthma treatment without talking to their physicians.