Preserving blood – why we need to look at new options
Posted: 22 February 2016 | Biopharma Group | No comments yet
Blood transfusions have been used successfully since the early 1900s, and blood banks have been in use since 1914. After donation, whole blood is separated into several components for processing and storage under different conditions, and one of these components is the red blood cell fraction.

How freeze drying is enabling improvements
Freeze drying potentially offers a long term storage solution for red blood cells without requiring refrigeration and allows rapid reconstitution of the lyophilised product at the point of use, making the freeze dried form suitable for use in both clinical environments and trauma sites.However red blood cells are challenging entities to lyophilise, proving sensitive to various


Water ice crystals on frozen blood sample
types of structural, oxidative and chemical damage during both freeze drying and the subsequent rehydration processes. Research performed by Biopharma Group‘s independent research and consultancy division BTL in collaboration with the University of Cambridge achieved a survival rate of 96% when freeze drying and rehydrating red blood cells, using a specially selected preservative formulation containing trehalose whereasthe level of Haemoglobin oxidation was at 60%, meaning overall the process was not viable. An alternative formulation was also tried which gave undetectable oxidation levels, but only an 85% survival rate.
It is believed thaSNAL Logot a limitation of the formulations trialled is the fact that the 

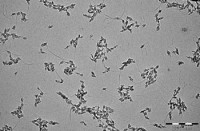

Figure 2. TEM micrograph of biomimetic apatite nanocrystals used in this RBC cryopreservation study. (size bar = 200 nm)
In these recent studies, freeze-thawing of red blood cells in phosphate buffered saline (PBS) has been compared with cells freeze-thawed in PBS-trehalose, apatite-trehalose and apatite-trehalose-saline solutions. The survival rate of the cells was found to increase in the order of formulations listed, where PBS-trehalose was the worst and apatite-trehalose-saline was the best. This suggests that the apatite may be allowing the trehalose to enter the cells, and testing is currently in progress to validate this theory. While there are still many hurdles to overcome, Biopharma Group is optimistic about this approach, and confident about the future of freeze drying red blood cells and the benefits this can bring to medicine.
In addition to researching new techniques for freeze drying red blood cells,Biopharma Group is also pleased to offer experience in freeze drying a wide range of blood products such as plasma and clotting factors. We have developed freeze drying cycles for several customers, achieving significant reductions in cycle time and improved control of residual moisture content compared to the existing cycles, and look forward to future challenges in this field.
Due to the independent nature of our research division, we are able to offer impartial consultancy and collaboration on many differing projects. To discuss how Biopharma can help you or to learn more the latest developments in freeze drying red blood cells, please contact us on +44 (0)1962 841092 or [email protected]




