How the use of placebos in freeze-drying enables cost efficiencies in process & cycle development
Posted: 19 February 2016 | Biopharma Group | No comments yet
A placebo is a simulated or otherwise medically ineffectual treatment for a disease or other medical condition intended to make the recipient believe they are receiving something they are not in order to establish whether the true active agent has a noticeable remedy to the ailment it is intended to treat against the control…


When using placebos in freeze-drying, whilst the active pharmaceutical ingredient (API) is absent the primary goal is not for the placebo to act as a decoy but rather to mimic as closely as possible the actual product in terms of its thermal and freeze‑drying behaviour.
Reasons for using placebo
There are various reasons to use placebo formulations in freeze-drying research and development, the most common being restrictions in use of the API due to high cost or low availability and restrictions due to high toxicity of the API.
Considerations in placebo selection
The first priority in placebo selection is ensuring that it displays characteristics as near as possible to the physiochemical properties of the actual product in terms of its freezing and freeze-drying behaviour. This can be achieved either by simply removing the API (if present in very low concentrations) or by replacing the API with a molecule that will simulate its presence in the formulation in terms of how the overall behaviour of the formulation is affected.
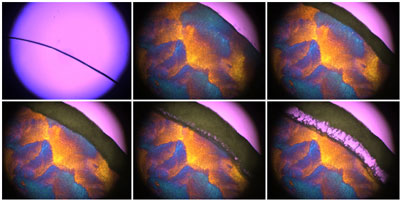

Extra care must be taken in choice of the simulant to ensure that it does not alter any significant events shown by the product. Patapoff and Overcashier1 showed that a placebo version of their formulation exhibited completely different freezing and drying characteristics from the API containing product, with the placebo drying significantly quicker. In this case the active protein was present in significant concentrations relative to the excipients and was replaced in the placebo formulation by a commonly used sugar. This illustrates the difficulties in selecting a placebo that acts as a suitable thermal simulant in formulations where the API has a dominant role in determining the freeze-drying of the product. Therefore, careful consideration must be given to the selection of an appropriate placebo formulation and whether the use of a placebo is actually a viable option. Another important factor to ensure is that the overall weight volume concentration of the placebo matches that of the product in order to duplicate the product’s resistance during drying. This is because a higher concentration formulation will give a denser structure of dried solute solution, giving a greater resistance to vapour flow. A placebo formulation that is less concentrated may dry quicker and give misleading results that cannot be applied to the product.
Use of placebos in characterisation studies for establishing critical cycle parameters
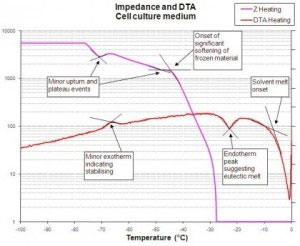

The first stage of developing a suitable freeze-drying cycle involves characterising the product to establish its inherent critical temperatures such as collapse or eutectic melt, and frozen state changes such as glass transition (Tg) or crystallisation events. Use of a placebo formulation to establish critical processing parameters for the product through the use of freeze-drying microscopy, impedance and differential thermal analysis or differential scanning calorimetry is not normally recommended as the API may well have a significant effect on the critical temperatures. Cycle development using these methods is possible if the API is present in only insignificant amounts and a placebo version of the product can be created by just omitting the API.
If the API is present in more significant quantities and likely to exert an effect upon critical temperatures, it may be possible to create a placebo which acts as a thermal simulant. For example, if the API is a protein it may be possible to replace this with a routine protein such as HSA. If a placebo is to be used during cycle development or validation activities it is recommended that the placebo also be characterised to verify the suitability of its use as a thermal simulant.
Use of placebos in Cycle Development
Placebos are commonly used in freeze-drying cycle development to bulk out trays so that only some vials need contain API due to possible restrictions in use due to cost or availability. Here a few vials containing API will be scattered amongst a tray of placebo vials. If an appropriate placebo has been selected any evidence of collapse or other processing deficiencies seen in the placebo vials should be representative of the actual product. If the placebo has been shown to closely match the product in its freeze-drying behaviour it may be possible to perform the cycle development programme entirely with the use of placebo. This approach would need to be retrospectively validated through performing a processing run on the actual product using the developed cycle to verify its suitability.
Use of placebos in Freeze-Dryer Transfer / Scale Up


In order to conserve the levels of API, placebo formulations are widely used in freeze-drier validation or engineering runs when scaling up production and/ or transferring a cycle to a new freeze-dryer.
For these activities several engineering runs are usually carried out for validation purposes before a full load of product is processed in the existing or new freeze-dryer. For these runs the batch can be entirely comprised of placebo or can be mostly placebo with some vials containing API scattered amongst them. If shelf mapping is available and scattered API is being used it would generally be recommended to place the actual product on any hot or cool spots in order to ensure that when it comes to production the cycle is robust enough to ensure product safety even with any temperature differentials across the freeze-dryer shelves. However, if the placebo closely matches the actual product thermal and freeze drying characteristics this may not be necessary. Following successful trials with placebo it would be advisable, where possible, to perform at least one final run using a load at least partially containing the actual product before commencing full production in order to fully validate the transfer or scale up activities.
In conclusion, placebos can be an essential part of developing and validating a suitable freeze‑drying process from initial characterisation through to cycle development and scale up to production. Careful consideration must be given to the selection of an appropriate placebo, however, in order to mimic as closely as possible the properties of the actual product. BTL regularly works with placebos for its global client base at all stages of the process development and is uniquely positioned to independently assist with the selection of a suitable placebo and undertake the requisite work.
References
- Overcashier, D., Patapoff, T., Hsu. C. (1999) Lyophilisation of protein formulation in vials: Investigation of the relationship between resistance to vapour flow during primary drying and small-scale product collapse. Journal of Pharmaceutical Sciences 88:688.
To find out more about using placebos in product characterisation and cycle development, please contact David Banks, Senior Scientist at Biopharma Technology Ltd. – [email protected] or +44 (0)1962 841092.





