NICE consults on dry eye treatment, ciclosporin, and asks for more information from the company
Posted: 24 June 2015 |
NICE has today published preliminary recommendations on ciclosporin (Ikervis) for treating severe keratitis in adults with dry eye disease…
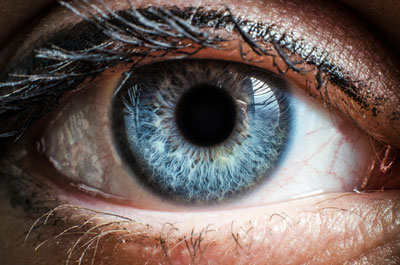

The National Institute for Health and Care Excellence (NICE) has today published preliminary recommendations on Santen Pharmaceutical’s ciclosporin (Ikervis) for treating severe keratitis in adults with dry eye disease which has not improved despite treatment with artificial tears, and has asked for more information from the company.


Dry eye disease is chronic inflammation of the eyes caused by reduced tear production or excessive tear evaporation. It can be triggered by a number of factors, including dry or air-conditioned environments, auto-immune diseases, and the adverse effects of some medications. Symptoms include irritation and redness in the eyes, blurred vision, and a sensation of grittiness or a foreign body in the eye.
Santen asked to provide more information on the cost and clinical effectiveness of ciclosporin
The independent Appraisal Committee noted that it had not been presented with evidence on the relative cost and clinical effectiveness of ciclosporin compared with established clinical practice. The Committee concluded that it needed additional evidence from the company to inform its decision-making, which should include:
An indirect comparison of the clinical effectiveness of ciclosporin plus corticosteroids (if needed) and artificial tears, and that of corticosteroids (if needed) and artificial tears.
An economic model comparing the cost effectiveness of ciclosporin plus corticosteroids (if needed) and artificial tears, with that of corticosteroids (if needed) and artificial tears.
Professor Carole Longson, Director of the Centre for Health Technology Evaluation at NICE said, “Severe dry eye disease can be painful and can have a significant negative effect on day-to-day life for people with the condition. Unfortunately, because of gaps and uncertainties in the evidence submitted by the company, NICE’s independent committee was minded not to recommend ciclosporin for this condition. The next step is for the company to submit further information requested by the Committee which will then be considered at its next meeting in August.”
Final guidance is expected to be published in September 2015.
Related organisations
National Institute for Health and Clinical Excellence (NICE), Santen Pharmaceutical Co




