Tecan and RECIPE announce co-marketing agreement for automation of LC-MSMS IVD kits
Posted: 26 August 2014 | | No comments yet
With clinical laboratories increasingly turning to high throughput LC-MSMS analysis, laboratory automation specialist Tecan and RECIPE Chemicals and Instruments GmbH have announced a co-marketing agreement for automation of LC-MSMS IVD kits…
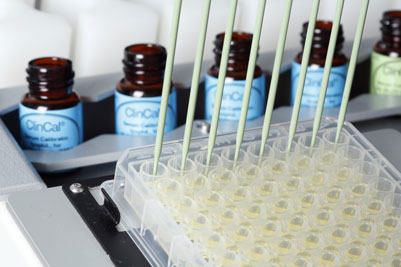

With clinical laboratories increasingly turning to high throughput LC-MSMS analysis, laboratory automation specialist Tecan and RECIPE Chemicals and Instruments GmbH, a leading manufacturer of IVD/CE diagnostic products, have announced a co-marketing agreement for automation of LC-MSMS IVD kits. Visitors to Booth #1 at MSACL 2014 EU in Salzburg, Austria, from the 2nd to the 5th of September, will have the opportunity to discover how Tecan’s flexible Freedom EVO® platform offers walkaway automation of the RECIPE ClinMass® LC-MSMS Complete Kits* for the analysis of vitamin D2/D3 and immunosuppressants. The automated process has the advantage of eliminating the potential for manual handling errors and increasing the speed of sample preparation three-fold compared to manual protocols, significantly improving turnaround times while enhancing reproducibility, traceability and sample security.


James O’Brien, Head of Clinical Diagnostics at Tecan, commented: “We are delighted to enter into this co-marketing agreement with RECIPE. Tecan’s extensive experience in laboratory automation and RECIPE’s expertise in the development and production of LC-MSMS IVD kits complement each other perfectly, offering customers the best of both worlds.”
For more information, visit Booth #1 at MSACL 2014 EU, or go to http://www.tecan.com/lcms




