Stem cells: from bench to drug development
Posted: 6 August 2014 | Lonza | No comments yet
Working in stem cell research can be both immensely rewarding and notoriously challenging. One of the most prominent challenges is taking what are often exciting new advances in stem cell biology made in the research lab, and translating these into viable, impactful therapies for patients.
This move from scientific discovery to therapy development requires a completely different approach than that applied to basic research. For example, in the lab it’s possible to make frequent minor tweaks to your experimental design: adjusting media components, trying new reagents, testing different substances/stimuli for cell differentiation etc. However, when you move over to a current good manufacturing practices (cGMP) manufacturing environment, you no longer have such ‘on the fly’ freedom. Instead you are required to have a robust and reproducible operating procedure firmly in place; you have to abide by stringent regulations with regards to the selection of reagents you can make use of; there are additional levels of quality control and assurance checks to pass; and of course there is the need to produce product in a GMP-compliant manufacturing suite.

Robust cell reprogramming
This list of cGMP requirements can be a tall order for many labs currently, or soon to be, tackling clinically focussed research. This problem has been recognized by specialist companies serving the Biotech and Pharma markets, driving the development of end-to-end services for people looking to translate their exciting lab work into real clinical applications. Lonza currently occupies a unique position in that they have an extensive research product portfolio in both Biotech and Pharma, providing primary and adult stem cells, pluripotent stem cells (PSCs), and all associated cell generation and culture methods.
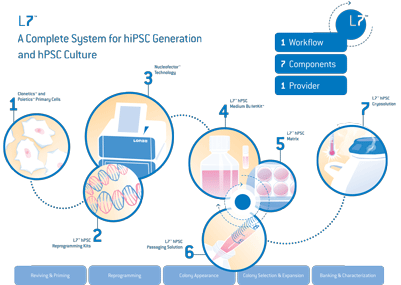
A great illustration of this comprehensive approach to working with stem cells is the generation of induced pluripotent stem cells (iPSCs). Human PSCs have the ability to generate any of the 220+ cell types found in the human body, and thus have great innate potential not just for basic research, but also drug discovery and cell replacement therapies. In order to make use of iPSCs in clinical research, they have to be of clinical grade quality. Many research labs aren’t set up for clinical grade cGMP manufacturing due to a lack of adequate facilities, quality systems or infrastructure. This led to the Lonza team adapting their non-viral 4D-NucleofectorTM Transfection Technology to meet higher quality standards (with cGMP solution kits and title 21 CFR part 11 compliant software) for use in a clinical grade manufacturing environment. Together with their L7™ hPSC Culture System (xeno-free and cGMP-compatible), this represents an excellent approach for the reprogramming and culturing of clinical grade iPSCs.
Addressing the needs of research
Since Shinya Yamanaka and John B. Gurdon were jointly awarded the Nobel Prize in 2012 for demonstrating that mature cells can be reprogrammed to become pluripotent, there has been a huge market-driven demand for efficient reprogramming methods. In response to this new drive towards clinical grade iPSC-generation, Lonza pioneered cGMP compatible solutions, equipment and reagent kits that are optimized to work as a complete system, so that those at the bench could start exploring clinically relevant stem cell research using a human PSC culture.
The real value of a complete systems approach to stem cell research is that it allows for the reproducible generation and maintenance of stem cells under defined conditions, eliminating the need to continually adjust a protocol. This leads to better time management and streamlined workflows, allowing for a seamless transition from bench to clinical development. The generation of robust, clinically relevant stem cells will have a major impact on the future of regenerative medicine. The ISSCR conference highlighted many of the areas where stem cell research is making real strides, such as the control of pluripotency, modelling diseases with iPSCs and the role of stem cells in cancer. These areas hold great promise for medical innovation and will hopefully yield results that contribute to the development of new and improved disease therapies.
If you would like to find out more about the systems we have developed for those working in stem cell biology, please visit http://www.lonza.com/L7.





