OLYSIO™ (simeprevir) receives marketing authorisation in the European Union for the treatment of adults with hepatitis C genotype 1 and 4 infection
Posted: 19 May 2014 | | No comments yet
Janssen-Cilag International NV today announced that its next generation protease inhibitor OLYSIOTM (simeprevir) has been granted marketing authorisation by the European Commission for the treatment of adults with genotype 1 and 4 chronic hepatitis C…
Janssen-Cilag International NV today announced that its next generation protease inhibitor (PI) OLYSIOTM (simeprevir) has been granted marketing authorisation by the European Commission (EC) for the treatment of adults with genotype 1 and 4 chronic hepatitis C (CHC), in combination with other medicinal products, which includes1:
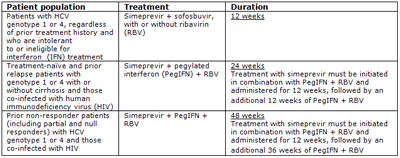

This marketing authorisation represents a significant milestone in the development of new triple therapy hepatitis C (HCV) treatment options for genotype 1 and 4 patients. It also includes simeprevir as part of an all oral 12-week IFN-free direct-acting antiviral (DAA) regimen with or without RBV, in genotype 1 or 4 patients, who are intolerant to or ineligible for IFN treatment.1
“The EC marketing authorisation for simeprevir is a great milestone as it adds an important new treatment option for patients, demonstrating the continued role of triple therapy in the treatment of HCV. In addition, the introduction of an all oral, 12-week interferon-free treatment regimen provides a new option for sustained virologic response in HCV patients with genotypes 1 or 4 intolerant to or ineligible for interferon-based treatment,” said Thomas Stark, Medical Director, Janssen EMEA.
HCV represents a major global public health concern. There are an estimated nine million people2 living with HCV in Europe which, if untreated, can cause severe damage to the liver, including cirrhosis and hepatocellular carcinoma (HCC). HCV represents a leading cause of liver transplantation in Europe.3 Whilst the number of patients being newly diagnosed with HCV is declining, it takes approximately 20 – 30 years for symptoms to appear, with HCV cases expecting to peak between 2030 and 2035.4,5
Dr Andrew Ustianowski, Chair of the British Viral Hepatitis Group and Consultant in Infectious Diseases at North Manchester General Hospital, commented: “The treatment environment in hepatitis C infection is evolving rapidly. Simeprevir is a well-tolerated and efficacious addition to our therapies against hepatitis C, and is a very welcome development for both those with genotype 1 and those with genotype 4.”
The EC marketing authorisation for simeprevir with PegIFN + RBV is based on a clinical trial programme involving three pivotal Phase 3 studies, with over 1000 patients. The trials, QUEST-1, QUEST-26 and PROMISE7, explored the use of simeprevir in combination with PegIFN + RBV in treatment-naïve patients and patients who have relapsed after prior interferon-based treatment. All three studies met their primary endpoints and demonstrated that simeprevir, in combination with PegIFN + RBV, achieves significant sustained virological response rates when compared with PegIFN + RBV alone.
The EC marketing authorisation for the combination of simeprevir and sofosbuvir also contains results from the Phase 2 study, COSMOS, in treatment-naïve patients. This was based upon prior null responder and treatment-naïve patients.8
Simeprevir is taken once-daily for 12 weeks, with treatment-naïve and prior-relapser patients receiving pegylated interferon and ribavirin for 24 weeks, and for 48 weeks total by those shown to be prior non-responder patients (including partial and null responders)1. It is generally well tolerated, with the most common adverse events reported in clinical trials (incidence ≥ 5%) including nausea, rash, pruritus, dyspnoea, blood bilirubin increase and photosensitivity reaction.1
In March 2013, simeprevir was approved for the treatment of genotype 1 HCV in Japan, in Canada in September 2013, and the U.S. in November 2013, with the most recent approval occurring in Russia in March 2014. Following the EC marketing authorisation, it is anticipated that simeprevir will be available across a number of European Union countries, in conjunction with reimbursement, in the second half of 2014.
References:
- Olysio SmPC. Accessed March 2014.
- Hatzakis A et. al. The state of hepatitis B and C in Europe: report from the hepatitis B and C summit conference. Journal of Viral Hepatitis, 2011:18,1-16.
- European Association for the Study of the Liver. EASL The Burden of Liver Disease in Europe. Available from http://www.easl.eu/medias/EASLimg/Discover/EU/54ae845caec619f_file.pdf. Accessed April 2014.
- Menzin J et al. BMC Health Services Research 2012; 12(459).
- Rein DB et al. Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Abstract Digestive and Liver Disease 2011. Available at: http://www.sciencedirect.com/science/article/pii/S1590865810001702 Accessed March 2014.
- Foster GR et al. Simeprevir (TMC435) with peginterferon/ribavirin for the treatment of chronic HCV genotype 1 infection in treatment-naïve European patients in the QUEST 1 and QUEST 2 Phase III studies. Abstract 1127. Poster presentation at the European Association for the Study of the Liver 2014.
- Forns X et al. The PROMISE study, Simeprevir (TMC 435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infection in European patients who relapsed after previous interferon-based therapy. Abstract 013. Oral presentation the European Association for the Study of the Liver 2014.
- Lawitz M et al. The COSMOS cohort 2 study, abstract presented at the European Associate for the Study of the Liver (EASL) 2014.




