FDA approves novel therapy for chronic skin condition
Posted: 21 April 2025 | Catherine Eckford (European Pharmaceutical Review) | No comments yet
The US approval could improve outcomes for eligible patients with previously limited options for managing the inflammatory skin condition.
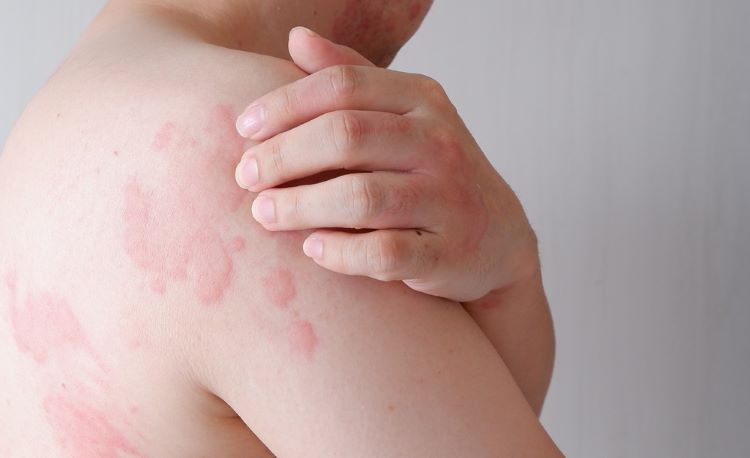

For the first time in over ten years, the US Food and Drug Administration (FDA) has approved a new targeted treatment for chronic spontaneous urticaria.
Sanofi and Regeneron Pharmaceuticals collaborated to develop Dupixent® (dupilumab). Under the FDA’s new authorisation, the therapy is indicated for individuals aged 12 years and over with the chronic inflammatory skin disease, who still experience symptoms despite taking histamine-1 (H1) antihistamine medication.
Why is the FDA’s decision to approve Dupixent as a new treatment for chronic spontaneous urticaria significant?
“approval of this treatment offers [chronic spontaneous urticaria] patients more options and the chance to control their disease”
“This FDA approval provides a new treatment option to help address the underlying drivers of these severe and recurring signs and symptoms. Dupixent has the potential to improve outcomes for CSU patients who previously had limited treatment options,” stated Dr Alyssa Johnsen, PhD, Global Therapeutic Area Head, Immunology and Oncology Development at Sanofi.
Pivotal clinical trials show that Dupixent can “help patients significantly reduce the hallmark symptoms of intense itch and unpredictable hives associated with this disease,” shared Dr George Yancopoulos, PhD, Board co-Chair, President and Chief Scientific Officer at Regeneron, and a principal inventor of Dupixent.
The FDA’s new approval of Dupixent is based on Phase III data from Study A and Study C that investigated the therapy for the skin condition. However, Study B, intended to provide additional safety data, was not appliable in the FDA’s decision for Dupixent, as it did not meet the primary endpoint in the US of reduction in ISS7 compared to placebo at 24 weeks.
Data from Study A and Study B were published in The Journal of Allergy and Clinical Immunology.
These trials demonstrated that Dupixent reduced itch severity and urticaria activity for participants compared to placebo at 24 weeks. Findings also showed that the monoclonal antibody increased the likelihood of participants attainting well-controlled disease or complete response compared to placebo at 24 weeks.
Other recent regulatory market approvals for Dupixent
In Nov 2024, Dupixent was granted approval in Europe to treat another inflammatory condition. The European Medicines Agency (EMA) authorised its use for paediatric patients with eosinophilic esophagitis.
Related topics
Big Pharma, Biologics, Biopharmaceuticals, Clinical Development, Clinical Trials, Data Analysis, Drug Development, Drug Markets, Drug Safety, Industry Insight, Regulation & Legislation, Research & Development (R&D), Therapeutics









